IRON and STEEL
Historical Review
Befor I'll talk about modern iron making, a short historical review should show you how important iron and steel has been being.
Iron tools and weapons were allready used by the prehistorical man, but the iron was not man-made, it has an meteoric orgin. Anyway, iron was used for various purposes thousend of years ago.
The time of the first man-made iron is still unknown, because the smelting process was certainly a result of chance, but the basic know-how of iron making is the same:
while heating up to high temperatures the ore must be in contact with the hot charcoal and
the reduction must take place in the absence of air.
Today we know 2 basic kinds of iron making:
the direct reduction and
the indirect reduction.
The 1st direct reduction process takes place between 1350 BC and 1100 BC. Simple hearths were used to win malleable iron which could then be forged by hammering. The big disadvantage was and still is, that it was a soft material, but they early found out that quenching mades it much harder. They also found out that iron with a carbon content above 2.06% could not be forged or rolled, but it could be casted into moulds. (This takes place the 1st time in China about 200 BC.)
To gain iron from the indirect reduction process the first simple blast furnaces had to be built. Principial the indirect reduction operates as follows:
In an initial semlting process the oxygen content of the iron was minimized, the result being pig iron.
During the 2nd process pig iron was purified of carbon and other elements, the result being wrouth iron.
The indirect reduction process had been first used in Belgium about 1620.
Today iron is primary gained with the indirect reduction process, so it will be expained at the chapter "blast furnace".
Basic materials
Necessary material groups for the iron and steel making processes are:
coal/coke,
iron ore,
fluxes and
oxygen
The most commen iron ores are magnetite (Fe3O4, 72.4%), hematite (Fe2O3, 69.94%) and siderite (FeCO3, 48.2%). Depending on the position of the ore in relation to the surface the ore get mined in open pits or underground. After mining the iron has to be seperated from the waste material. This task is achieved by magentic means or heavy media seperation.
Another very important material is the coke, which is gained by destructiv distillation of bituminous coal. Destructiv distillation means that the process takes place in the absence of air. It also should be said, that not all kinds of coal could be charged to coke directly.
The two main jobs of the coke are:
to work as a fuel for the combustion (temperaturs of about 2000°C are needed) and
the carbon frees the iron from the oxygen, the so called reduction.
The 3rd group of important materials are the fluxes. One of their tasks is to seperate the iron from the mechanical mixture, which could be rather difficult, as the gangue frequently has a high melting point. The flux also makes the process a two components system, which leads to the fact that some materials are easier to melt.
Their main job is to work as a reactant with those elements, which are unwanted in the iron. The two types of fluxes are acid and basic fluxes, depending on their chemical composition. The most frequently used fluxes are limestone (CaCO3) and silica (SiO2).
The blast furnace
Blast furnaces work according to the counter current principle, this means that the burden, which is charged in at the top, slowly moves downwards as the process goes on. When the burden reaches a temperatur over 500°C the reduction starts and intensifies to closer it gets to the combustion zone. Depending on the position in the furnace, the reaction from the iron takes place with carbonmonoxide (CO), carbon (C) or hydrogen (H). Reactions also take place with manganese (Mg), silicon (Si), phosporous (P) and free oxygen (O), but these reactions are usually unwanted.
The sinking burden gets preheated by the ascending gases before the chemical reactions start.
At the bosh the first slag developes at about 1000°C. At this point the iron is reduced to sponge iron and metallic iron. After passing the tuyeres, the charge reaches its highest temperatur. Now slag floats on the top of the hot metal, because of his specific weight.
Slag and iron are generally tapped every 3 houres.The iron is usually delivered to the steelmaking plants or transported to the foundry for producing grey castings.
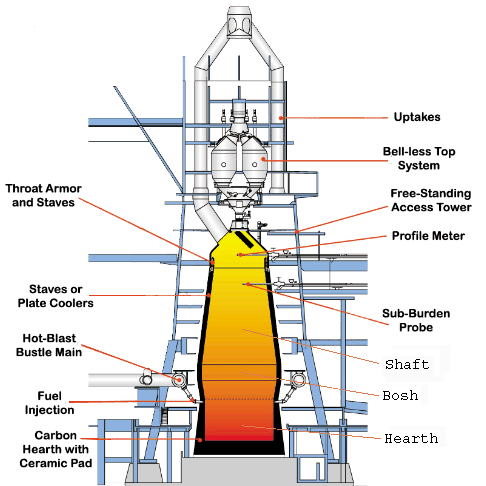
The following picture should show
you the important parts of an blast furnace:
General Ancillary Equipment, including
Free-standing shell and tower
Taphole equipment
Optimized for access and economy
Furnace instrumentation and valves
Designed for future shell replacement
Hot-metal and slag-handling systems
Furnace Top Slag Treatment
Advanced burden charging equipment
Heavy duty throat armor castings
Options of wet or dry slag granulation
Production of quality product for the
Cast iron staves with independent cement industry cooling circuit
Hot-Blast Stoves Stave Cooling
Internal and external combustion
The most robust integral linings chamber designs
Copper staves for high heat flux zones
Cast iron Staves for maximum
High efficiency, low-emission ceramic
burners cost-effectiveness
Waste-heat recovery
Refractories Gas Cleaning
Silicon carbide in the bosh and belly
Dust recycling
Erosion-resistant alumina in the upper stack
High efficiency wet gas cleaning for secundary cleaning
Erosion-resistant carbon hearth walls with ceramic pad
Dustcatchers and cyclones for primary cleaning
Complete water treatment plants
Fuel Injection
Coal injection
Process Control
Fuel gas injection
Oil injection
Advanced sensors for monitoring in-furnace conditions
Cast House
Numerous heat and mass balance models
Flat floor for safe and open access
Stoves, burden and hearth-wear models
Dedusting and dust suppression
Online kinetic process model
Remote operation of cast house equipment
Expert system for closed-loop control
In-launder desiliconization and ladle desulfurization
The steelmaking process
Steel is iron containing less than 2.06% of carbon. Some of the main differences are that steel can be rolled, forged and casted.
In the steelmaking process the wrouth iron has to undergo a process of oxidation in order to reduce the content of carbon (C), silicon (Si) and phosphorous (P). But during this process other elements could be added to change the proberties, depending on the add material, of the steel.
The three most important steel production processes will be mentioned here:
The open hearth furnace process
Up to the 1960's open hearth furnaces were widely used to produce ordinary grades of steel. Today they are becoming less and less economical - althought 30% of world steel productions is still carried out by SM (Siemens Martin) furnaces, mainly in the USA and the USSR.
Electric furnace:
In an electric furnace the "charge", mostly scrap, must first be melted. These days scrap ist sometimes replaced by sponge iron which has been produced in a direct reduction plant. This melting is achieved by an electric arc between graphite electrodes and the charge. Near the electrodes the temperatur can rise over 3000°C. In the newest types of electric furnaces, oxygen lances blow in oxygen in order to shorten the process. The latest desings also include jet burners, which blow in fuel in order to reduce the melting period. This process is still used in the production of common steel and steel for special purposes, which alloy elements such as chromium, nickel and molybdenum.
The LD process:
In this process, developed in the Linz and Donawitz works in the 1940's, hot metal is turned into steel. The hot metal needed in this process is first homogenized in mixers. Then transporting ladles deliver it to the LD vessel, which is lined with refractory bricks.
No further heat from outside is necessary, as the temeratur required develops in process of oxidation of various chemical elements enclosed in the hot metal itself. The oxygen needed for this process of oxidation is blown in with water cooled lances at a pressure of 8-12 bar from above (distance 0.5 to 1m). In this process of oxidation a temperatur of over 1600°C is reached. The excess heat is used for melting the scrap which has been added and which comprises up to 30% of the total charge of the furnace. Lime and other additives are put in to help form slag. Tap to tap time is about 40 minutes, the blowing process itselfts takes from 12 to 18 minutes, depending on the grade of steel.
After processing the furnaces are tapped and the crude steel is emptied into transporting ladles. At every tapping samples must be taken in order to be analysed in the quality control department.
Further processing of steel
The liquid steel can be transported to teeming bays, where it is cast into moulds to form ingots. Thereby we different between rimmed and killed ingots, depending on the chemical structur across the section.
It can also be processed in continous casters. Therfor killed steel is necessary. In the continous casting process the steel is cast into water cooled copper moulds which are open at the bottom. The shape of the mould determines the cross section of the slabs, billets or blooms.
To let the steel solidify, the head of the dummy bar is equipped with rail and scrap pieces. At the end of the casting train, oxyacetylene cutters or shears cut them to lengh required at the rolling mills.
But the steel can also be rolled. Therefor the slabs, billets or blooms, depending on the required profil, have to be heatet up to a temperatur of about 1250°C
Depending on the endproduct, different types of rolling mills exist.
Haupt | Fügen Sie Referat | Kontakt | Impressum | Nutzungsbedingungen
